K Vs Q

Comparing q vs k example.
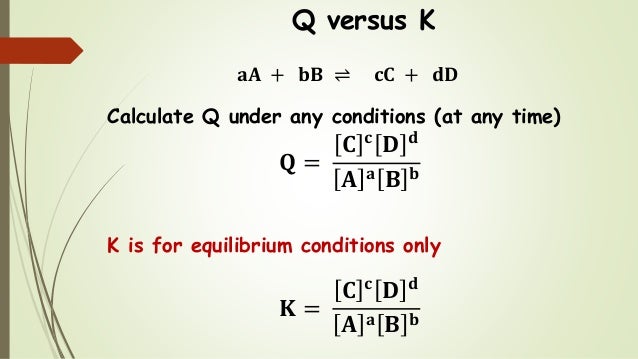
K vs q. A simple relationship between k c and the reaction quotient known as q c can help. The 10 k covers both the fourth quarter and the entire year large accelerated filers and accelerated filers have 40 days to release their 10 qs. Y h k m l g v s n q and o. This just says that if you mix a and b a will react to become b and b will react to become a this equation also states that one molecule of a reacts to give one molecule of b this will be an important part of the concept.
This works for any pair and overcards even 22 vs 34 for example. Using either the initial concentrations or initial activities of all the components of the reaction the progression of an reaction can easily be determined. Video transcript voiceover a one litre reaction vessel contains 1 2 moles of carbon monoxide 1 5 moles of hydrogen gas and 2 0 moles of methanol gas. This report is filed if there are any changes or developments to a business that didn t make the 10 q or 10 k reports.
The reaction quotient q expresses the relative ratio of products to reactants at a given instant. In addition to filing annual reports on form 10 k and quarterly reports on form 10 q public companies must report certain material corporate events on a more current basis. For a given general chemical equation. Truthfully the odds aren t exactly 50 50 in these scenarios but they are close enough that they are considered a coin flip.
Revenue fares you ll see that expertflyer gives us a ton more information including upgrade and award fare codes. Form 8 k is the current report companies must file with the sec to announce major events that shareholders should know about. Q and k consider a simple chemical system including just two compounds a and b. The most common flip situation you ll see or more likely be in is the classic pair vs.
The instructions for form 8 k describe the types of events that trigger a public company. This is considered an unscheduled document and may contain information. Eg ak vs 99 or aj vs 77. The form 10 q filing.
The main difference between k and q is that k describes a reaction that is at equilibrium whereas q describes a reaction that is not at equilibrium. To determine q the concentrations of the reactants and products must be known. Basic economy while these fare classes represent all the tickets you can buy directly with cold hard cash i e. Using le chatelier s principle.