K Vs Q Equilibrium
So what s gonna happen is in order to reach equilibrium our concentrations are going to shift to the right to get q closer.
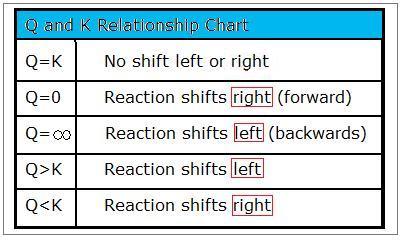
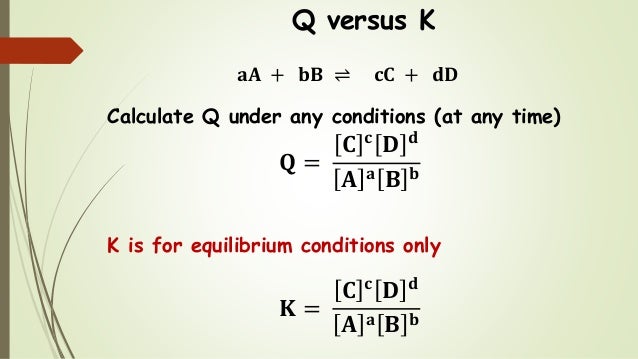
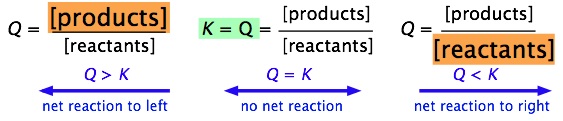
K vs q equilibrium. The main difference between k and q is that k describes a reaction that is at equilibrium whereas q describes a reaction that is not at equilibrium. And k is 14 5 so we ll say it s somewhere around here. It shows you how to determine in which direction the reactio. Aa bb rightleftharpoons cc dd tag 1.
5 q 0 033 so q k. The simulation includes five different reactions which each have three scenarios. Q and k often get mixed up since they have the same form. For example if we combine the two reactants a and b at concentrations of 1 mol l 1 each the value of q will be 0 1 0.
Notice that the value of q increases as soon as some more of the product nh 3 g is added to the equilibrium mixture. Factors that affect chemical equilibrium. Q k q k and q k. The goal of this web page is to help you understand this distinction between q and k and to let you explore the effect of changing the initial concentrations of a and b the value of k and the speed of the reaction on the final.
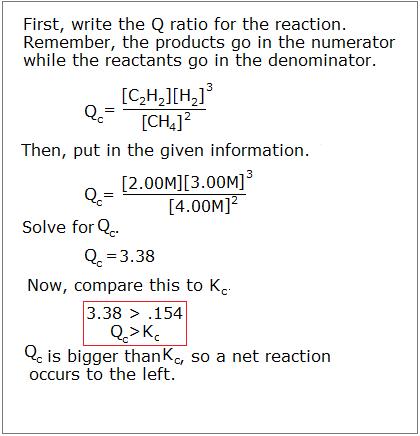
Comparing q vs k example. This chemistry video tutorial focuses on the reaction quotient q and the equilibrium constant k. The reaction quotient q. As a system approaches towards equilibrium q approaches towards k.
Since q k the reaction will shift to the right to reach equilibrium. 4 no it is not at equilibrium. So q we can put on our number line is somewhere around here. The reaction will shift to the right.
The value of q in relation to k serves as an index how the composition of the reaction system compares to that of the equilibrium state and thus it indicates the direction in which any net reaction must proceed. For a given general chemical equation. 7 q 12 so q k. In the second portion of the question the students will compare the value of q to the equilibrium constant k and predict which way the reaction will shift to reach equilibrium.
We can see that q is less than k on our number line. Introduction to reaction quotient qc. Our mission is to provide a free world class education to anyone anywhere. The reaction will shift to the left.
The only possible change is the conversion of some of. Comparing q vs k example. Q is a quantity that changes as a reaction system approaches equilibrium. For as long as the value of q is greater than k the reaction will proceed in the reverse.
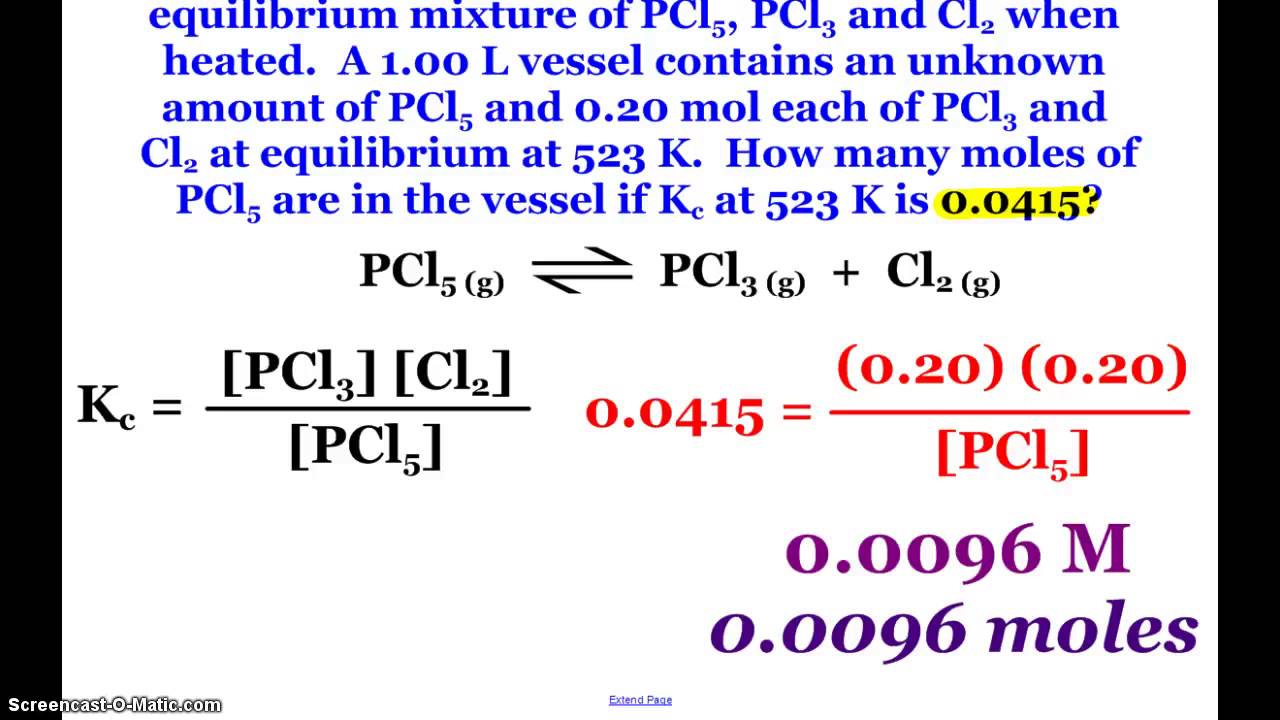
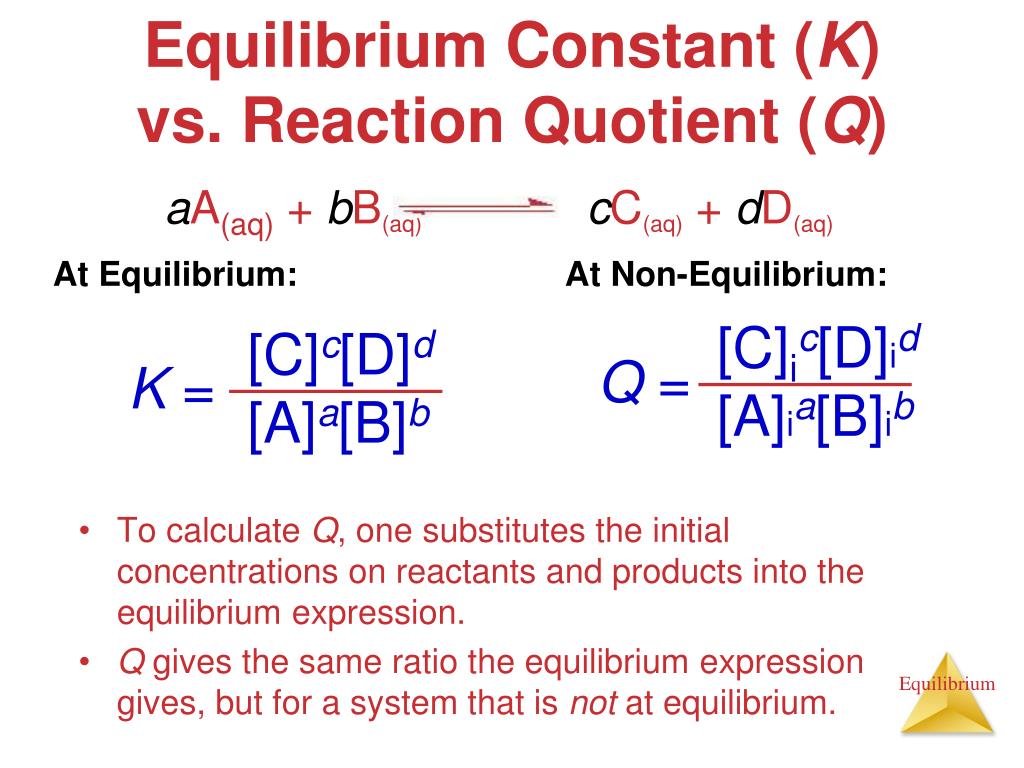
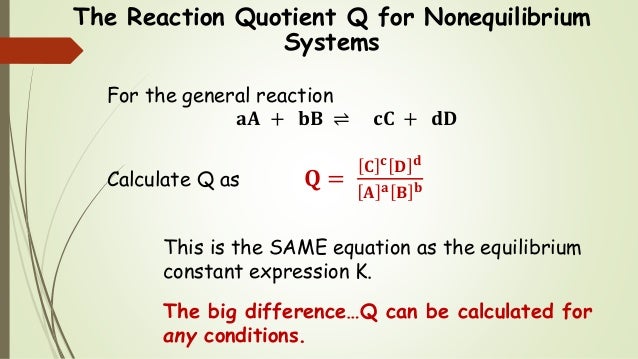
Q is the value we get any point away from equilibrium. To determine q the concentrations of the reactants and products must be known. They are both state functions the difference is k is the value of the mass action expression the concentrations or pressures of the products over the reactants when those concentrations or pressures are the values at equilibrium. 6 q 32 0 so q k.
K is the numerical value of q at the end of the reaction when equilibrium is reached.